Skin Test Devices
Skin Test Devices at a Glance
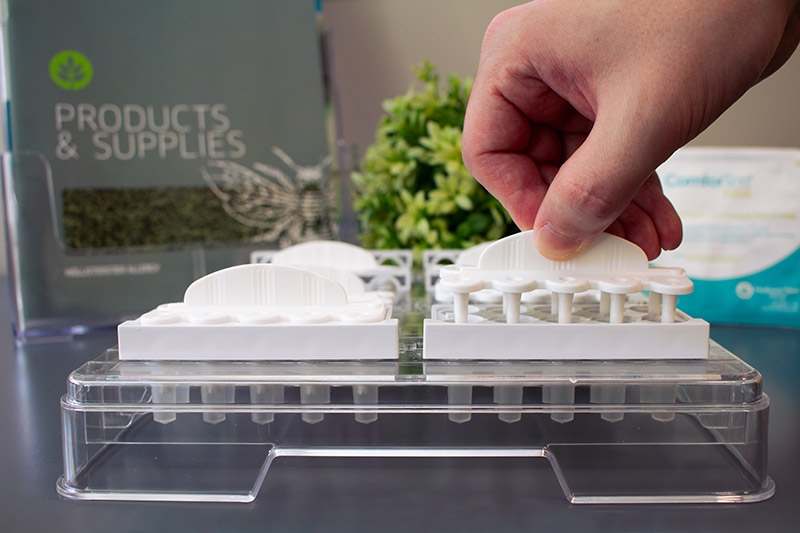
HollisterStier Allergy’s skin test device systems provide healthcare professionals with a quick, easy setup. Experience an efficient and reproducible testing experience with ComforTen® and Quintip®.
Designed with You in Mind
Our skin test device systems are designed to save you time and money. After receiving feedback from nurses in the allergy field, we designed:
- A translucent tray for easy antigen inspection
- Removable reservoirs to minimize mistakes
- Collars to prevent foreign material and evaporation
- 1.2mm lancet tips with depth control guards for reproducible results and minimal patient discomfort
HollisterStier’s skin test devices produce a 0mm reaction at the negative control site, allowing readings of 3mm+ to read positive.1,2,3,4
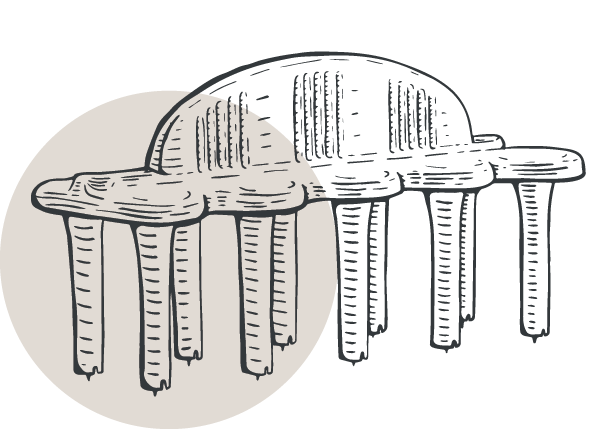
ComforTen® Multiple Skin Test System
See the ComforTen® Device in Action
Test ten extracts at once with the ComforTen Multiple Skin Test System®, available in a 30-hole or 60-hole tray. Enjoy all the benefits of our skin test device systems, along with optional-use spacers that increase testing capacity.
Watch this video to learn more about the skin test device system. You’ll discover how ComforTen® elevates the allergy testing experience and receive a demo on how easy it is.
Quintip® Individual Skin Test System
Customize Your Testing with Quintip® Devices
Easily integrate a single extract into your existing setup. With Quintip® you can enjoy all the benefits of the ComforTen® Device, including stainless steel lancet tips and a 1.2mm depth guard while streamlining your allergenic testing process.
ComforTen® and Quintip® fully comply with OSHA’s Needlestick Safety and Prevention Act. Refer to the package inserts for further information.
Resources
2Carr, W.W., et al. “Comparison of Test Devices for Skin Prick Testing” J. Allergy Clin. Immunol.; 116: 341-346, August 2005.
3Nelson, H.S., et al. “Evaluation of Devices for Skin Prick Testing” J. Allergy Clin. Immunol.; 101: 153-156, February 1998.
4Bernstein, L., et al. “Allergy Diagnostic Testing: An Updated Practice Parameter” Annals of Allergy, Asthma & Immunology.; 100: S1, S16-19, March 2008.
The foregoing references are studies on HollisterStier’s QUINTEST®/QUINTIP® skin test devices. Because ComforTen® technology is based on HollisterStier’s QUINTEST®/QUINTIP® skin test devices, HollisterStier believes these references apply to ComforTen® as well.